|
Professional Supplier Platform Company
|
Gold Index: 12313
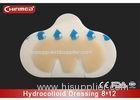
medical adhesive central venous catheter dressing
| Place of Origin: | Zhejiang, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
1,<spa
1,Polyurethane thin film, transparent, waterproof, air-permit, allows wound observation;
2,Paper frame design ,makes the stick more smooth and comfortable.
3,Paper tape attached, used as information record and better fixation on tubing or helps dressing removal.
Description:
Compare to Tegaderm
Application:
For fixing I.V catheter (basic)
Specification:
| Order Number | Size | Pcs/box | Boxes/Carton |
| CM17001 | 6*7cm | 100 | 40 |
| CM17001U | 6*7cm | 100 | 40 |
| CM17001R | 6*7cm | 100 | 40 |
| CM17002 | 10*12cm | 100 | 24 |
| CM17003 | 7*8.5cm | 100 | 24 |
| CM17004 | 8.5*10.5cm | 100 | 24 |
| CM17005 | 10*11.5cm | 100 | 24 |
| CM17006 | 4.4*4.4cm | 100 | 40 |
customize can be accept.
Usage:





User guide:
step1, Take out the dressing, peel off the release paper, and stick on the skin. Make sure skin is clean and dry before using.
step2, Make sure that I. V point is on the centre of the dressing, and place the incision part right towards I.V catheter.
Step3, Stick the dressing on skin natrually,do not drag it. Press the dressing slightly from the I.V point. Then peel off the paper frame from the tab design, and press the edge of the dressingsmoothly meanwhile.
step4, Smooth the dressing to enhance the fixing.
step5, Write down the name , bed No. and other neccessary information on the recording tape.
Factory show:

FAQ:
1,Q:Are you trade company or manufacture?
A:We are factory
2,Q:Where is your factory located? How can I visit?
A:Our factory is located in ningbo city zhejiang province china.You can flight to shanghai and then take train to ningbo city. warmly welcome to visit us
3,Q:How can I get some samples?
A:we will glad to offer you sample.
4,Q:what's the certification do you get?
A: CE,FDA and ISO13485 quality syst
Related Search
Find more related products in following catalogs on Hisupplier.com
Related Products

Company Info
Professional Supplier Platform Company [China (Mainland)]
Business Type:Manufacturer
City: Hong Kong Island
Province/State: Hong Kong
Country/Region: China (Mainland)